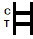NTENDED USE
The StrongStep® PROM test is a visually interpreted, qualitative immunochromatographic test for detection of IGFBP-1 from amniotic fluid in vaginal secretions during pregnancy. The test is intended for professional use to help diagnose the rupture of fetal membranes (ROM) in pregnant women.
INTRODUCTION
The concentration of IGFBP-1 (insulin-like growth factor binding protein-1) in amniotic fluid is 100 to 1000 times higher than in maternal serum. IGFBP-1 is not usually present in the vagina, but after rupture of fetal membranes, amniotic fluid with a high concentration of IGFBP-1 mixes with vaginal secretions. In StrongStep® PROM test, a specimen of vaginal secretion is taken with a sterile polyester swab and the specimen is extracted into Specimen Extraction Solution. The presence of IGFBP-1 in the solution is detected using a rapid test device.
PRINCIPLE
The StrongStep® PROM Test uses color immunochromatographic, capillary flow technology. The test procedure requires the solubilization of IGFBP-1 from a vaginal swab by mixing the swab in Sample Buffer. Then the mixed sample buffer is added to the test cassette sample well and the mixture migrates along the membrane surface. If IGFBP-1 is present in the sample, it will form a complex with the primary anti- IGFBP-1 antibody conjugated to colored particles. The complex will then be bound by a second anti- IGFBP-1 antibody coated on the nitrocellulose membrane. The appearance of a visible test line along with the control line will indicate a positive result.
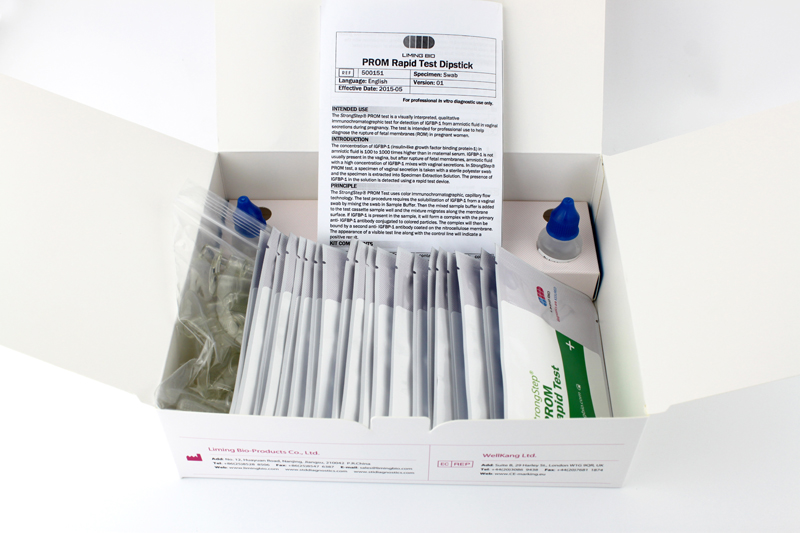
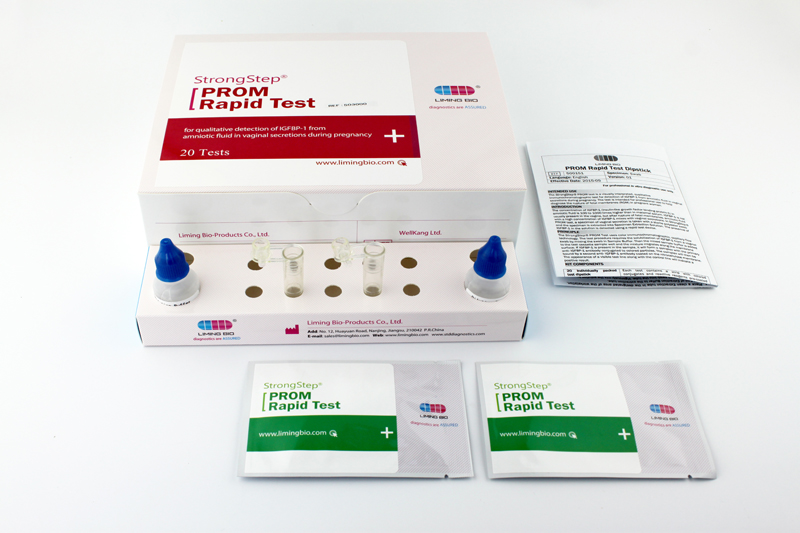
KIT COMPONENTS
|
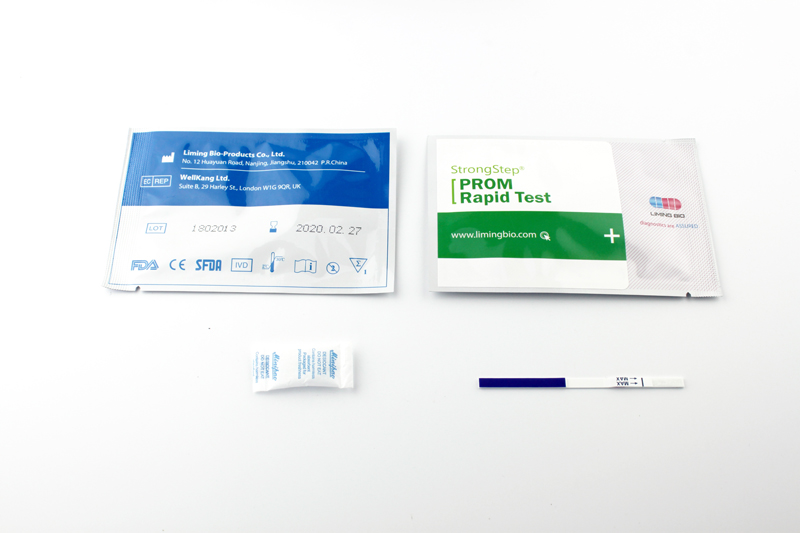
20 Individually packed test devices |
Each device contains a strip with colored conjugates and reactive reagents pre-coated at the corresponding regions. |
|
2 Extraction Buffer vial |
0.1 M Phosphate buffered saline (PBS) and 0.02% sodium azide. |
| 1 Positive control swab (on request only) |
Contain IGFBP-1 and sodium azide. For External control. |
| 1 Negative control swab (on request only) |
Not contain IGFBP-1. For external control. |
|
20 Extraction tubes |
For specimens preparation use. |
|
1 Workstation |
Place for holding buffer vials and tubes. |
|
1 Package insert |
For operation instruction. |
MATERIALS REQUIRED BUT NOT PROVIDED
| Timer | For timing use. |
PRECAUTIONS
■ For professional in vitro diagnostic use only.
■ Do not use after expiration date indicated on the package. Do not use the test if its foil pouch is damaged. Do not reuse tests.
■ This kit contains products of animal origin. Certified knowledge of the origin and/or sanitary state of the animals does not totally guarantee the absence of transmissible pathogenic agents. It is therefore, recommended that these products be treated as potentially infectious, and handled observing the usual safety precautions (do not ingest or inhale).
■ Avoid cross-contamination of specimens by using a new specimen collection container for each specimen obtained.
■ Read the entire procedure carefully prior to performing any tests.
■ Do not eat, drink or smoke in the area where the specimens and kits are handled. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow the standard procedures for proper disposal of specimens. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
■ Do not interchange or mix reagents from different lots. Do not mix solution bottle caps.
■ Humidity and temperature can adversely affect results.
■ When the assay procedure is completed, dispose the swabs carefully after autoclaving them at 121°C for at least 20 minutes. Alternatively, they can be treated with 0.5% sodium hypochloride (or house-hold bleach) for one hour before disposal. The used testing materials should be discarded in accordance with local, state and/or federal regulations.
■ Do not use cytology brushes with pregnant patients.
STORAGE AND STABILITY
■ The kit should be stored at 2-30°C until the expiry date printed on the sealed pouch.
■ The test must remain in the sealed pouch until use.
■ Do not freeze.
■ Cares should be taken to protect components in this kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipments, containers or reagents can lead to false results.
SPECIMEN COLLECTION AND STORAGE
Use only Dacron or Rayon tipped sterile swabs with plastic shafts. It is recommend to use the swab supplied by the kits manufacturer(The swabs are not contained in this kit, for the ordering information, please contact the manufacturer or local distributor, the cataloge number is 207000). Swabs from other suppliers have not been validated. Swabs with cotton tips or wooden shafts are not recommended.
■ A sample is obtained using a sterile polyester swab. The sample should be collected prior to performing digital examination and/or transvaginal ultrasound. Take care not to touch anything with the swab before taking the sample. Carefully insert the tip of the swab into the vagina toward the posterior fornix until resistance is met. Alternatively the sample can be taken from the posterior fornix during a sterile speculum examination. The swab should be left in the vagina for 10-15 seconds to allow it to absorb the vaginal secretion. Pull the swab out carefully!.
■ Put the swab to the extraction tube, if the test may be run immediately. If immediate testing is not possible, the patient samples should be placed in a dry transport tube for storage or transport. The swabs may be stored for 24 hours at room temperature (15-30°C) or 1 week at 4°C or no more than 6 month at -20°C. All specimens should be allowed to reach a room temperature of 15-30°C before testing.
PROCEDURE
Bring tests, specimens, buffer and/or controls to room temperature (15-30°C) before use.
■ Place a clean Extraction tube in the designated area of the workstation. Add 1ml of Extraction Buffer to the extraction tube.
■ Put the specimen swab into the tube. Vigorously mix the solution by rotating the swab forcefully against the side of the tube for least ten times (while submerged). Best results are obtained when the specimen is vigorously mixed in the solution.
■ Squeeze out as much liquid as possible from the swab by pinching the side of the flexible extraction tube as the swab is removed. At least 1/2 of the sample buffer solution must remain in the tube for adequate capillary migration to occur. Put the cap onto the extracted tube.
Discard the swab in a suitable biohazardous waste container.
■ The specimens extracted can retain at room temperature for 60 minutes without affecting the result of the test.
■ Remove the test from its sealed pouch, and place it on a clean, level surface. Label the device with patient or control identification. To obtain a best result, the assay should be performed within one hour.
■ Add 3 drops (approximately 100 µl) of extracted sample from the Extraction Tube to the sample well on the test cassette.
Avoid trapping air bubbles in the specimen well (S), and do not drop any solution in observation window.
As the test begins to work, you will see color move across the membrane.
■ Wait for the colored band(s) to appear. The result should be read at 5 minutes. Do not interpret the result after 5 minutes.
Discard used test tubes and Test Cassettes in suitable biohazardous waste container.
NTERPRETATION OF RESULTS
|
POSITIVE RESULT:
|
Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T). |
|
NEGATIVE RESULT:
|
Only one colored band appears in the control region (C). No apparent colored band appears in the test region (T). |
|
INVALID RESULT:
|
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor. |
NOTE:
1. The intensity of the color in test region (T) may vary depending on the concentration of aimed substances present in the specimen. But the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
QUALITY CONTROL
■ Internal procedural controls are included in the test. A colored band appearing in the control region (C) is considered as an internal positive procedural control. It confirms sufficient specimen volume and correct procedural technique.
■ External procedural controls may provided(on request only) in the kits to ensure that the tests are functioning properly. Also, the Controls may be used to demonstrate proper performance by the test operator. To perform a positive or negative control test, complete the steps in the Test Procedure section treating the control swab in the same manner as a specimen swab.
LIMITATIONS OF THE TEST
1. No quantitative interpretation should be made based on the test results.
2.Do not use the test if its aluminum foil pouch or the seals of the pouch are not intact.
3.A positive StrongStep® PROM test result, although detecting the presence of amniotic fluid in the sample, does not locate the site of the rupture.
4.As with all diagnostic tests, results must be interpreted in the light of other clinical findings.
5.If rupture of fetal membranes has occurred but the leakage of amniotic fluid has ceased more than 12 hours before the specimen is taken, IGFBP-1 may have been degraded by proteases in the vagina and the test may give a negative result.
PERFORMANCE CHARACTERISTICS
Table: StrongStep® PROM Test vs. Another brand PROM Test
| Relative Sensitivity: 96.92% (89.32%-99.63%)* Relative Specificity: 97.87% (93.91%-99.56%)* Overall Agreement: 97.57% (94.42%-99.21%)* *95% Confidence Interval |
|
Another brand |
|
||
|
+ |
– |
Total |
|||
|
StrongStep® PROM Test |
+ |
63 |
3 |
66 |
|
|
– |
2 |
138 |
140 |
||
|
|
65 |
141 |
206 |
||
Analytic sensitivity
The lowest detectable amount of IGFBP-1 in the extracted sample is 12.5 μg/l.
Interfering Substances
Care must be taken not to contaminate the applicator or cervicovaginal secretions with lubricants, soaps, disinfectants, or creams. Lubricants or creams may physically interfere with absorption of the specimen onto the applicator. Soaps or disinfectants may interfere with the antibody-antigen reaction.
Potential interfering substances were tested at concentrations that might be reasonably found in cervicovaginal secretions. The following substances did not interfere in the assay when tested at the levels indicated.
| Substance | Concentration | Substance | Concentration |
| Ampicillin | 1.47 mg/mL | Prostaglandin F2 | 0.033 mg/mL |
| Erythromycin | 0.272 mg/mL | Prostaglandin E2 | 0.033 mg/mL |
| Maternal Urine 3rd Trimester | 5% (vol) | MonistatR (miconazole) | 0.5 mg/mL |
| Oxytocin | 10 IU/mL | Indigo Carmine | 0.232 mg/mL |
| Terbutaline | 3.59 mg/mL | Gentamicin | 0.849 mg/mL |
| Dexamethasone | 2.50 mg/mL | BetadineR Gel | 10 mg/mL |
| MgSO4•7H2O | 1.49 mg/mL | BetadineR Cleanser | 10 mg/mL |
| Ritodrine | 0.33 mg/mL | K-YR Jelly | 62.5 mg/mL |
| DermicidolR 2000 | 25.73 mg/mL |
LITERATURE REFERENCES
Erdemoglu and Mungan T. Significance of detecting insulin-like growth factor binding protein-1 in cervicovaginal secretions: comparison with nitrazine test and amniotic fluid volume assessment. Acta Obstet Gynecol Scand (2004) 83:622-626.
Kubota T and Takeuchi H. Evaluation of insulin-like growth factor binding protein-1 as a diagnostic tool for rupture of the membranes. J Obstet Gynecol Res (1998) 24:411-417.
Rutanen E-M et al. Evaluation of a rapid strip test for insulin-like growth factor binding protein-1 in the diagnosis of ruptured fetal membranes. Clin Chim Acta (1996) 253:91-101.
Rutanen E-M, Pekonen F, Karkkainen T. Measurement of insulin-like growth factor binding protein-1 in cervical/vaginal secretions: comparison with the ROM-check Membrane Immunoassay in the diagnosis of ruptured fetal membranes. Clin Chim Acta (1993) 214:73-81.
GLOSSARY OF SYMBOLS
|
|
Catalog number |
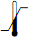 |
Temperature limitation |
 |
Consult instructions for use |
|
Batch code |
 |
In vitro diagnostic medical device |
 |
Use by |
 |
Manufacturer |
 |
Contains sufficient for <n> tests |
 |
Do not reuse |
 |
Authorized representative in the European Community |
 |
CE marked according to IVD Medical Devices Directive 98/79/EC |
||