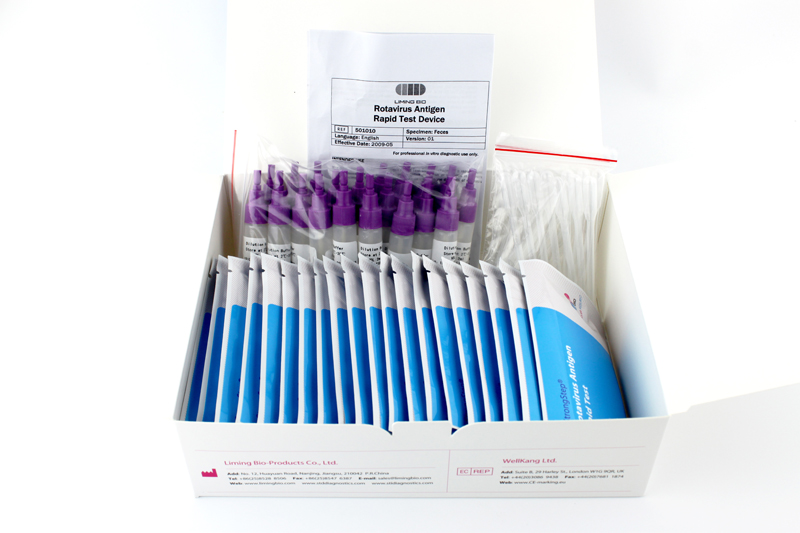
INTRODUCTION
Rotavirus is the most common agent responsible for acute gastroenteritis, mainly in young children. Its discovery in 1973 and its association with infantile gastro-enteritis represented a very important advancement in the study of gastroenteritis not caused by acute bacterial infection. Rotavirus is transmitted by oral-fecal route with an incubation period of 1-3 days. Although specimens collected within the second and fifth day of the illness are ideal for antigen detection, rotavirus may still be found while diarrhea continues. Rotaviral gastroenteritis may result in mortality for populations at risk such as infants, the elderly and immunocompromised patients. In temperate climates, rotavirus infections occur mainly in the winter months. Endemics as well as epidemics affecting some thousand people have been reported. With hospitalized children suffering from acute enteric disease, up to 50% of the analyzed specimens were positive for rotavirus. The viruses replicate in the
cell nucleus and tend to be host species-specific producing a characteristic cytopathic effect (CPE). Because rotavirus is extremely difficult to culture, it is unusual to use isolation of the virus in the diagnosis of infections. Instead, a variety of techniques have been developed to detect rotavirus in feces.
PRINCIPLE
The Rotavirus Rapid Test Device (Feces) detects rotavirus through visual interpretation of color development on the internal strip. Anti-rotavirus antibodies are immobilized on the test region of the membrane. During testing, the specimen
reacts with anti-rotavirus antibodies conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there is
sufficient rotavirus in the specimen, a colored band will form at the test region of the membrane. The presence of this colored band indicates a positive result, while its absence indicates a negative result. The appearance of a colored band at the
control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
KIT COMPONENTS
| Individually packed test devices | Each device contains a strip with colored conjugates and reactive reagents pre-coated at the corresponding regions. |
| Specimens dilution tube with buffer | 0.1 M Phosphate buffered saline (PBS) and 0.02% sodium azide. |
| Disposable pipettes | For collecting of liquid specimens |
| Package insert | For operating instructions |
MATERIALS REQUIRED BUT NOT PROVIDED
| Timer | For timing use |
| Centrifuge | For treatment of specimens in special circumstances |